
Mpox: WHO Approves Administration of Jynneos Vaccine for Adolescents
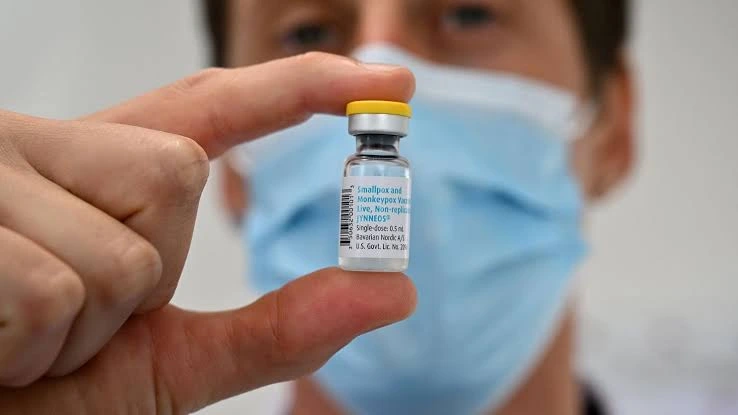
On Monday, the World Health Organisation (WHO) announced its approval of Bavarian Nordic’s Jynneos vaccine for adolescents aged 12 to 17. This group is considered particularly vulnerable to mpox outbreaks. The vaccine was prequalified by the WHO on October 8, 2024, following its earlier approval for adults.
The approval comes after the European Union authorised the vaccine for adolescents in September. The WHO declared mpox a global public health emergency in August, after a new strain of the virus spread across many countries.
Bavarian Nordic is set to begin clinical trials to assess the vaccine’s safety for children aged two to 12. The trials are expected to start in October. Meanwhile, the U.S. FDA has approved the vaccine for adults.
Another mpox vaccine, LC16, developed by Japan’s KM Biologics, is already approved for use in children but requires a specialised needle for administration.
Read More:
- GTBank Faces Backlash From Customers as Transition to New Banking System Causes Prolonged Delay
- FAAN Union Leaders Accuse Aviation Minister of Secret Airport Concession Deals
About The Author
Related Articles
The AFCON Final in Morocco and the Controversies That Followed
The Africa Cup of Nations final between hosts Morocco and Senegal ended...
ByWest Africa WeeklyJanuary 20, 2026Mali’s Transition Leader Attends Swearing-In of Guinea’s President Mamadi Doumbouya
Mali’s President of the Transition, General Assimi Goïta, represented the country in...
ByWest Africa WeeklyJanuary 19, 2026Malian Army Conducts Successful Surveillance Operation in Mopti Region
The Malian Armed Forces have carried out a successful territorial surveillance operation...
ByWest Africa WeeklyJanuary 19, 2026Niger’s Security Forces Record Major Gains Against Armed Groups
Niger’s Defence and Security Forces have reported significant results following a week...
ByWest Africa WeeklyJanuary 19, 2026












Leave a comment