
NAFDAC Alerts Nigerians on Counterfeit Breast Cancer Drug
The National Agency for Food and Drugs Administration and Control (NAFDAC) has issued a public alert regarding a counterfeit batch of Phesgo 600mg/600mg/10ml injection, an essential medication used in the treatment of breast cancer.
The counterfeit product, labelled with batch number C5290S20, was reported to the agency by a doctor at Lagos University Teaching Hospital (LUTH-NSIA) after being presented by a patient for administration.
According to NAFDAC, the Marketing Authorisation Holder (MAH), Roche, confirmed the falsified nature of the product following an investigation.
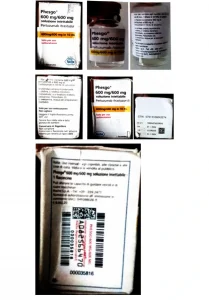
Although no physical sample was available for chemical analysis, images provided revealed significant discrepancies between the counterfeit and genuine materials, including:
A non-existent batch number in Roche’s database.
Inconsistent language on the packaging.
Missing “Basilisk” and incorrect “Bollino” date.
Mismatched tamper-evidence labels compared to genuine Roche products. The counterfeit product falsely claimed Roche as the manufacturer with a stated manufacturing site of “Roche S, P.A.” However, genuine Phesgo® 600mg/600mg is manufactured by F. Hoffman-La Roche Limited in Switzerland.
Read Also: NAFDAC Shuts Down 150 Shops in Aba’s Eziukwu Market Over Tons of Fake and Expired Products
Shettima Misuses Nigerian Taxpayer’s Money, Flies Official Jet to Attend Wedding
- source: NAFDAC
NAFDAC urges healthcare providers and the public to remain vigilant and avoid the use of products that appear suspicious.
About The Author
Related Articles
MultiChoice Pumps GH¢200 Million Into Ghana’s Creative Industry
MultiChoice Ghana has invested more than GH¢200 million into the country’s creative...
ByWest Africa WeeklyMarch 5, 2026FIFA Confirms DR Congo Playoff Spot, Ending Nigeria’s World Cup Dream
Nigeria’s hopes of qualifying for the 2026 FIFA World Cup have come...
ByWest Africa WeeklyMarch 5, 2026Ghanaians to Travel to St Kitts and Nevis Without Visa
Ghanaian citizens will soon be able to travel to the Caribbean nation...
ByWest Africa WeeklyMarch 5, 2026Ghana Records Sharp Drop in Inflation, Lowest Since 2021
Ghana’s inflation rate dropped to 3.3 percent in February 2026, marking the...
ByWest Africa WeeklyMarch 5, 2026











Leave a comment